What Does Colloidal Mean? Part 2
- Peter L. Reynolds, Ph.D.
- Mar 17, 2015
- 3 min read
You may wonder why, as the particle size goes down, the area goes up. A simple way to explain this is, as the particles size goes down, less of the mass of the particle is deep within the inside of the particle. Thus, more of the mass of the particle is on or close to the surface. As a result, more of the particle can react with some outside stimulous like heat. Therefore, down to 5nm, the smaller the particle, the greater the amount of potential reactive surface area.
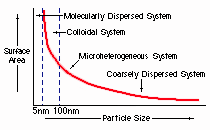
You may have noticed that the curve doesn't cross the 5nm line. Particles which are smaller than 5nm cease to have surface area as we commonly think of it. Instead, we are now dealing with molecules and atoms where surface, in the traditional sense, doesn't exist. In this case, another form of physics (Einsteinian) dealing with electron clouds, bonds, shells, etc., comes into play and substances take on other, often more profound characteristics. This explains why a molecular dispersion, such as silver nitrate, has quite different characteristics than a colloidal dispersion, such as colloidal silver. Silver nitrate stains, burns, and is quite toxic. On the other hand, colloidal silver does not stain, does not burn, and is not toxic.
Besides particles within the 5nm to 100nm range suspended in a medium, there are three other characteristics which all colloids exhibit. First, every colloid is heterogeneous (meaning: consisting of dissimilar ingredients or constituents, like silver and water). Second, every colloid is multiphasic (meaning: having more than one phase such as solid/liquid, gas/liquid, etc.). Third, the particles in every colloid are insoluble (meaning: the particles don't dissolve) in the medium but remain distinct particles suspended in the medium.). Each of these three characteristics interact with the others giving colloids their unique qualities.
Table 2 portrays a variety of colloidal systems using solids, liquids and gases.

As you may be surprised to find out, many materials associated with ordinary life, such as soap, many plastics and clouds are colloids. Even more astounding is the role colloids play in organic functions, such as digestion and excretion. Suffice it to say, we have met the colloids and the colloids are us (Sorry, that's not an exact quote.).
Colloids were also classified based upon the interaction of the particles with the medium, suspension, or solvent. The three main classifications are:
(1) lyophobic (medium-hating)
(2) lyophilic (medium-loving)
(3) association colloids.
In lyophobic colloids, the medium between the particles has little attraction for the particles. Therefore, these colloids are difficult to keep stable (meaning: remaining dispersed). Metal sols like collidal silver fall into this category.
Metal sols (colloidal silver) often owe their stability to like-electrically-charged particles. Such charged particles repel each other like same-pole magnets. Thus, if the size (mass) of the particles is small enough such that the pull of gravity is less than the repelling force, the particles will remain dispersed in the medium, indefinitely. Many high-grade (Super Tyndall Effect Colloidal Silver) metal sols fit this description.
If the size (mass) of the particles is so large that the pull of gravity is greater than the repelling force, the particles will eventually settle to the bottom of the container. Some metal sols that have larger particles use stabilizers (such as gelatin, egg-white, starch, etc.) added to the medium to slow particles settling to the bottom. Thus, these sols temporarily appear to be stable because the particles settle more slowly through the viscous medium. Some low-grade metal sols fit this description.
In lyophilic colloids, the particles are attracted to the medium or suspension. These colloids are more stable than lyophobic (colloidal silver) colloids because the medium helps the particles remain dispersed. Often, lyophilic colloids are made up of high molecular weight compounds. Such lyophilic colloids are found in animal and plant fluids.
The molecules of association colloids have a hydrocarbon chain with a hydrophilic (water-loving) "head" group and a hydrophobic (water-hating) "tail" group. When dissolved in water, the molecules form clusters called micelles. The micelles keep the head groups in contact with the water, while protecting the tail groups from the water within the micelle. The interior of the micelles dissolves oily material and keeps it in a stable suspension. Detergents and soaps are common examples of association colloids.
I hope you are enjoying learning about colloids and colloidal silver as much as I am. Lets take a break here. Stay tuned, more articles coming soon!





Comments