What Does Colloidal Mean? Part 1
- Aug 19, 2014
- 2 min read
By Peter L. Reynolds, Ph.D.
I must admit, it didn't mean a thing to me when I first heard the term. It wouldn't surprise me if you weren't a little rusty on the subject, too, unless you were going to graduate school over 60 years, ago. If that's the case, you might be feeling a bit rusty in other areas as well.
The word, colloidal, comes from the word, colloid. The term, colloid, was actually coined by an English chemist named Thomas Graham in the 1860's.

From his and other's insights, a colloid is defined as being a substance composed of extremely small (1) particles, but larger than most molecules, suspended in some (2) medium that is a gas, a liquid or a solid. The combination of (1) particles and (2) medium in a colloid is referred to as a Colloidal System.
How small is extremely small? Colloidal Systems have particles that range in size from 5 nanometers (a nanometer (nm) is a billionth of a meter or one thousandth of a micron. So 5nm = .005 microns or 5 thousandths of one millionth of a meter) to 100nm (100 nanometers = .1 micron = one tenth of one millionth of a meter). Particles of this size are almost unimaginable. For years, no light microscope could even begin to magnify such a small particle up to a size that was discernable. It was not until the invention of scanner and transmission electron microscopes, we could "see" such small particles. So, the particle size range for all colloids is from 5nm to 100nm.
Systems that have particles generally smaller than .005 microns or 5nm are classified as Molecular Systems and exhibit markedly different characteristics than do Colloidal Systems. This is because particles that are smaller than 5nm start to respond like molecules, which don't have surface area in the same way we normally think of surface area (when it comes to colloids, the more surface area, the more effective). Instead, at the molecular level we're dealing with electron clouds. It's a whole new physics (Einsteinian) when we move into a Molecular System.
For systems that have particles larger than .1 micron or 100nm, one moves into Microheterogeneous Systems, towards Coarsely Dispersed Systems. Again, these systems have quite different characteristics from Colloidal Systems.
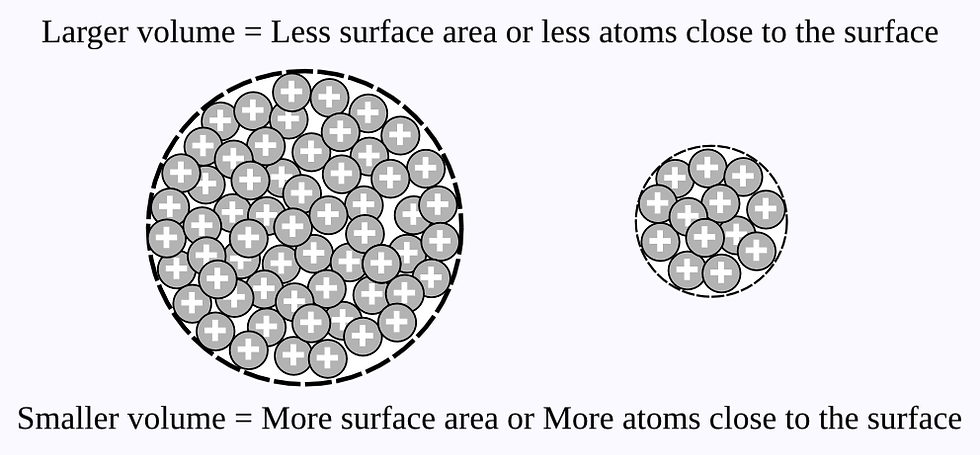
Early researchers found that the greater the surface area, the more effective the suspension. The finer the particles with surface area, the more reactive it will be. In the case of colloids, the more reactive, the more effective.
You may wonder why, as the particle size goes down, the area goes up. A simple way to explain this is, as the particles size goes down, less of the mass of the particle is deep within the inside of the particle. Thus, more of the mass of the particle is on or close to the surface. As a result, more of the particle can react with some outside stimulous like heat. Therefore, down to 5nm, the smaller the particle, the greater the amount of potential reactive surface area.
Ok, lets take a break! if you are not tired however, you can go to part 2.





Comments